More information is available at t4phagecures.com
]]>https://onlinelibrary.wiley.com/doi/full/10.1002/smsc.202400580
]]>Full Article: https://www.nature.com/articles/s41467-023-38364-1
]]>For a more detailed reading, you can visit the original article here.
]]>“An Effective Needle-Free Mucosal Phage T4-COVID Vaccine” presents a comprehensive study on a novel, needle-free COVID-19 vaccine. This vaccine, based on bacteriophage T4, is designed for mucosal administration, particularly through intranasal delivery. The study highlights the vaccine’s ability to induce strong mucosal and systemic immune responses, including robust antibody production and T-cell activation. The vaccine shows efficacy against various SARS-CoV-2 variants and offers complete protection in animal models. Notably, this T4-based vaccine remains stable at ambient temperature, addressing a significant challenge in vaccine distribution and storage. ]]>
https://www.biorxiv.org/content/10.1101/2022.04.28.489809v1
The next logical step, will be ‘challenge’ in non-human Primates?
LONG-COVID: One-in-three have complained that they are ‘just not right’ … so you may be able to think of this (nasal spray) T4 Phage construction as a “Therapeutic”, as well as prevention.
Approximately 100 million Americans have contracted Coronavirus (components) either through vaccination or natural infection.
Reminder, that reports regarding the Pfizer “Paxlovid” antiviral pill, seems to allow regeneration/less kill rate of the resident CoV (soon after completing the course of 10 pills), which is very disappointing. Only 20 million courses were purchased. It takes 8 months to make a pill, according to reporting.
Generally people do not like needles (better compliance is needed); delivery in the US Mail; stable at room temperature; team is working on cGMP; may prove less shedding; may prove fewer re-infections; deserves T Cells distribution in various important tissues analysis; rapid turnaround times, as virulent types are discovered; moduler & flexibility, built in.
Random CoV variant waves are presenting every 4 to 6 months. We see no end to this reality.
Also, Government wants a UNIVERSAL FLU VACCINE …. https://grants.nih.gov/grants/guide/notice-files/NOT-AI-22-013.html
The same vaccine platform can serve as prevention vs many different diseases; simply exchange the antigens/epitopes.
Clark Tibbs, CEO
PhageVax, Inc.
Cell: 740.502.9010
Today we face the largest pandemic threats in modern history. The World Health Organization (WHO) has deemed the H1N1 Swine Flu an “unstoppable” worldwide virus. Some experts warn that viral mixes could trigger an even more deadly strain (source). With these prospects, what can be done?
What if there was a way not only to produce vaccine in a quarter of the time that most large vaccine labs manufacture vaccines, but also to do it with a mobile platform easily transported to the hardest hit pandemic hotspots? PhageVax, Inc. has that solution.
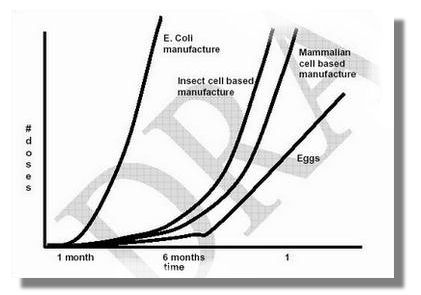
]]>- As noted in the recent Centers for Disease Control and Prevention community planning guidance1, the only intervention that can reasonably be expected to control an influenza pandemic is vaccination of a large fraction of the population with a strain-matched vaccine. Planners and modelers commonly assume that such a vaccine intervention would be similar to recent vaccination interventions for seasonal influenza, employing egg-based vaccine production technology. Thus analyses assume that pandemic influenza vaccine will not be available until four to six months after the onset of the pandemic, and that vaccine could not be produced faster than 4 million doses per week (corresponding to 100 million doses in 6 months). Under this view, several recent studies2-5 have analyzed how various non-vaccine interventions might be able to control an influenza pandemic until vaccine can be delivered. Other studies6 do not incorporate vaccination production and delivery because of the assumption that it would be too late.
- Alternative approaches to egg-based vaccine production would grow virus in bioreactors using mammalian cell cultures, insect cell cultures (e.g. NovaVax, Inc. and Protein Sciences, Inc.), or bacteria (e.g. PhageVax, Inc.). These technology developments might reduce the lag time between identification of the influenza strain and initial vaccine production capability, and might also allow higher US production rates. This analysis considers the potential of these new technologies to mitigate a pandemic.
- The effectiveness of a vaccine intervention to control an influenza pandemic will depend on 1) when the vaccine will first become available, 2) how many people can be treated per week once vaccination starts, 3) how effective the vaccine is, and 4) how many days following inoculation are required for immunity to build up. In addition, the impact of a vaccine intervention on the pandemic will depend on other interventions that are used in conjunction. This analysis compares the impact of vaccine distribution starting four months earlier than the seasonal influenza experience, comparing the impact of a range of vaccine production rates. It also compares the case of a two-dose course with a one-dose requirement. Most significantly, it examines the impact of early vaccine intervention used in conjunction with antiviral medication.
- If a vaccine can be produced four months earlier relative to egg-based seasonal influenza vaccine production, at a production rate sufficient to vaccinate 95% of the population within a one-month period, the impact on the pandemic would be enormous. In the absence of any other intervention, such a vaccine would reduce the mortality rate by a factor of five (from 614 deaths per 100,000 persons to 121 deaths per 100,000). Furthermore, if the existing strategic national stockpile (SNS) of 20 million courses of antiviral medication is used in conjunction with the vaccine intervention (for therapeutic treatment of diagnosed cases and prophylactic treatment of household members of diagnosed cases), the additional use of the early, rapidly produced vaccine would reduce the mortality from 550 per 100,000 to only 3 per 100,000, well below the mortality rate of seasonal influenza.
Read The Full Document with Graphical Data – Download
- As noted in the recent Centers for Disease Control and Prevention community planning guidance1, the only intervention that can reasonably be expected to control an influenza pandemic is vaccination of a large fraction of the population with a strain-matched vaccine. Planners and modelers commonly assume that such a vaccine intervention would be similar to recent vaccination interventions for seasonal influenza, employing egg-based vaccine production technology. Thus analyses assume that pandemic influenza vaccine will not be available until four to six months after the onset of the pandemic, and that vaccine could not be produced faster than 4 million doses per week (corresponding to 100 million doses in 6 months). Under this view, several recent studies2-5 have analyzed how various non-vaccine interventions might be able to control an influenza pandemic until vaccine can be delivered. Other studies6 do not incorporate vaccination production and delivery because of the assumption that it would be too late.
- Alternative approaches to egg-based vaccine production would grow virus in bioreactors using mammalian cell cultures, insect cell cultures (e.g. NovaVax, Inc. and Protein Sciences, Inc.), or bacteria (e.g. PhageVax, Inc.). These technology developments might reduce the lag time between identification of the influenza strain and initial vaccine production capability, and might also allow higher US production rates. This analysis considers the potential of these new technologies to mitigate a pandemic.
- The effectiveness of a vaccine intervention to control an influenza pandemic will depend on 1) when the vaccine will first become available, 2) how many people can be treated per week once vaccination starts, 3) how effective the vaccine is, and 4) how many days following inoculation are required for immunity to build up. In addition, the impact of a vaccine intervention on the pandemic will depend on other interventions that are used in conjunction. This analysis compares the impact of vaccine distribution starting four months earlier than the seasonal influenza experience, comparing the impact of a range of vaccine production rates. It also compares the case of a two-dose course with a one-dose requirement. Most significantly, it examines the impact of early vaccine intervention used in conjunction with antiviral medication.
- If a vaccine can be produced four months earlier relative to egg-based seasonal influenza vaccine production, at a production rate sufficient to vaccinate 95% of the population within a one-month period, the impact on the pandemic would be enormous. In the absence of any other intervention, such a vaccine would reduce the mortality rate by a factor of five (from 614 deaths per 100,000 persons to 121 deaths per 100,000). Furthermore, if the existing strategic national stockpile (SNS) of 20 million courses of antiviral medication is used in conjunction with the vaccine intervention (for therapeutic treatment of diagnosed cases and prophylactic treatment of household members of diagnosed cases), the additional use of the early, rapidly produced vaccine would reduce the mortality from 550 per 100,000 to only 3 per 100,000, well below the mortality rate of seasonal influenza.
WiredPRNews.com — US based company has developed a new vaccine production method for combating emerging infectious diseases – including the novel H1N1 swine flu and the deadly H5N1 avian flu. These various sub-types of Influenza may merge in the 2nd and 3rd Pandemic Waves and become easily transmissible from human to human and deadly. Newark, Ohio July 1, 2009 – PhageVax has created a novel platform to produce vaccines against infectious diseases – from pathogen identification to the scaled manufacture of vaccine – in one-quarter the time as current technologies. This process allows for millions of doses to be produced and distributed in a matter of 4 to 6 weeks for both civilian and military personnel. Traditional vaccine generation, using eggs or other cell-culture or other insect-cell media, typically takes from 4 to 6 months. In a time where pandemics can move around the globe at the speed of a jetliner, the ability to develop and manufacture large amounts of vaccine quickly is vital. A key concern is that within the time-frame of traditional vaccine manufacture the pathogen often has mutated, thus rendering the proposed [egg-based or cell-based] vaccines useless. Recent reports from Denmark show that the novel H1N1 was resistant to the anti-viral called Tamiflu. This may become a trend.
PhageVax’s Bacteriophage-DNA Vaccine Platform represents a “just in time” manufacturing process for combating rapidly evolving infectious diseases. The company’s platform technology allows for production of a vaccine (from the initial identification of a pathogen to a cGMP lot of vaccine capable of protecting tens of millions of people) in 4 to 6 weeks; literally in one-quarter the time of traditional methods and at a fraction of the US Tax-payer’s cost compared to egg-based Flu Vaccines. Why is this important? On June 11, the World Health Organization declared a Phase Six global novel H1N1 (swine) Flu Pandemic. A Phase Six pandemic declaration is based on the sustained worldwide spread of said virus. In addition, it has been learned the deadly H5N1 virus involved in the Flu outbreaks (in Egypt) is Tamiflu-resistant, although the virus was never exposed to Tamiflu (the primary anti-viral stockpiled against a flu epidemic in the US). The novel H1N1 may merge with the deadly H5N1 from Southeast Asia and/or merge with the deadly H5N1 from Egypt. Each of these H5N1 viruses are distinct from each other, thus raising the complexity of any human protection. The traditional vaccine production time-frame; flexibility; volume simply cannot respond quickly enough to combat these unforeseen pathogenic organisms. PhageVax has made arrangements for clinical trials at three research labs to confirm its findings and is currently negotiating with the National Institute of Health, and the CDC and the FDA for implementation and production of vaccines to combat swine flu, avian flu and malaria.
Research suggests that the vaccines may be sufficiently effective where one dose may protect against infection from wild-type disease.
 ]]>
]]>